Recently, Prof. Guan Xingang’s team published a research paper entitled “Engineering SIRPα cellular membrane-based nanovesicles for combination immunotherapy” in Nano Research (Impact Factor: 10.269), a TOP CAS Q1 journal. The first author of the paper is Mingyue Wang, a jointly educated master’s degree student; Prof. Guan Xingang is the first corresponding author of the paper; and Taizhou University is the first corresponding institution of the paper.
Cancer immunotherapy is a novel cancer treatment method by activating the body’s own immune system. In recent years, immune checkpoint inhibitors (ICIs), represented by PD-1 and PD-L1 antibodies, have become the most popular cancer treatment drugs worldwide. The approved ICIs have been used alone or in combination for more than a dozen clinical cancer treatments, substantially improving patient survival. However, the low single-agent response rate, immune-related adverse events and tumor resistance of existing ICIs have limited their further clinical application. The development of highly effective ICIs based on new targets has become a hot frontier in current antitumor research.
Signal-regulatory protein α (SIRPα) is a phagocytic signaling checkpoint expressed on myeloid cells such as macrophages, dendritic cells and neutrophils. Normal cells expressing CD47 molecules bind to SIRPα on the surface of macrophages and release the "don't eat me" signal, thus protecting them from immune system attack. Tumor cells, by highly expressing CD47 ligand molecules on their surface and binding to SIRPα receptors on macrophages, prevent the phagocytosis of tumors by macrophages and evade immune system recognition and attack. Several phagocytosis checkpoint inhibitors, including CD47 antibody, SIRPα antibody, and SIRP-Fc protein, have already entered phase I/II clinical trials in cancer.
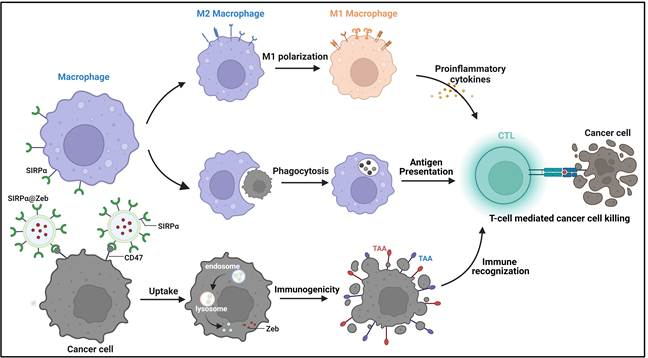
Schematic diagram of the mechanism of multi-pathway activation of anti-tumor immune response by SIRPα cell membrane vesicle nanodrug
In this resaerch, we designed and prepared a cell membrane nanovesicle expressing SIRPα receptor to specifically block the CD47-SIRPα signaling axis, promote macrophage phagocytosis, promote M2-type macrophages to M1 polarization, and activate anti-tumor immune response. In addition, considering that tumors evade immunosurveillance through low (or no) expression of tumor-associated antigen (TAA), this study utilized SIRPα cell membrane vesicle endosomes loaded with Zebularine (Zeb), a DNA methylation enzyme inhibitor, to enhance melanoma-specific antigens (CD146 and TRP1) through Zeb. (Zeb enhances the expression of melanoma-specific antigens (CD146 and TRP1) and promotes tumor recognition by the immune system; uses the SIRPα receptor on the cell membrane vesicles to bind to the CD47 ligand of tumor cells, blocking the "don't eat me" signal between tumor cells and macrophages and promoting the processing of tumor antigens The study was conducted to improve cancer therapeutic efficacy through the release of pro-inflammatory factors from polarized M1-type macrophages and the activation of the body's anti-tumor immune response in multiple ways. This study provides important data to support the development of phagocytic checkpoint-based ICIs.
This research was performed in collaboration with Taizhou University, CAS Changchun Institute of Applied Chemistry, and Zhejiang University, and the research was funded by Taizhou University and the National Natural Science Foundation of China.
Link to the paper: https://doi.org/10.1007/s12274-023-5397-4