Recently, the team from School of Pharmaceutical and Chemical Engineering, led by Tong Xiaofeng, published a research paper titled “Transition from Kwon [4+2]- to [3+2]-cycloaddition Enabled by AgF-assisted Phosphine Catalysis” in Nature Communications. This is the second high-level paper on AgF-assisted tertiary phosphine catalysis research published by the team following their work in Angew. Chem. Int. Ed. 2024, 63, e20231518 earlier this year.
In the nearly 70-year history of research on tertiary phosphine catalysis, the core development strategy has been to use differently structured electrophilic reagents to form unique reactive dipolar ions A1-A5 (Figure-1a). In this study, the group took an innovative approach by using AgF additives to modify these traditional dipolar ion intermediates, thereby obtaining new reactivity. The specific research approach is shown in Figure 1b.
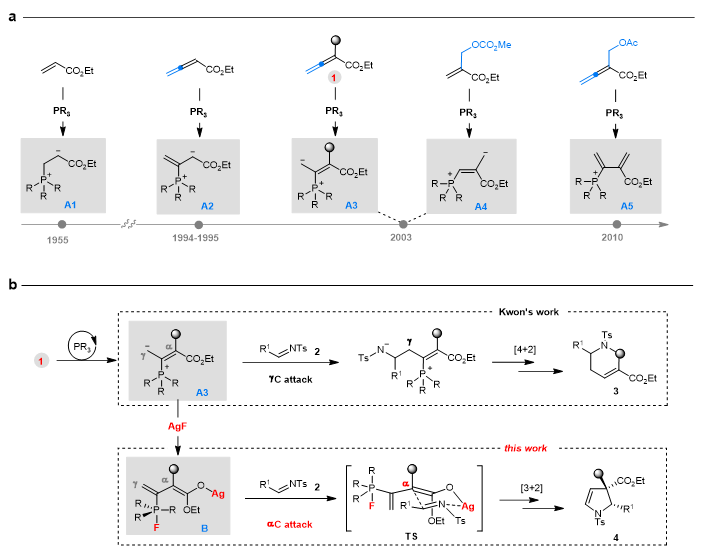
Figure 1: Research background and approach
In this study, the team applied the AgF-assisted strategy to 2-substituted allenyl esters 1. As early as 2003, Professor Kwon from Caltech reported the [4+2] cycloaddition reaction between allenyl ester 1 and imine 2 under tertiary phosphine catalysis. Under very similar reaction conditions, simply by adding equimolar amounts of AgF, the reaction between allenyl ester 1 and imine 2 was a [3+2] cycloaddition instead. More notably, this new [3+2] cycloaddition reaction exhibits excellent substrate generality and high stereoselectivity.
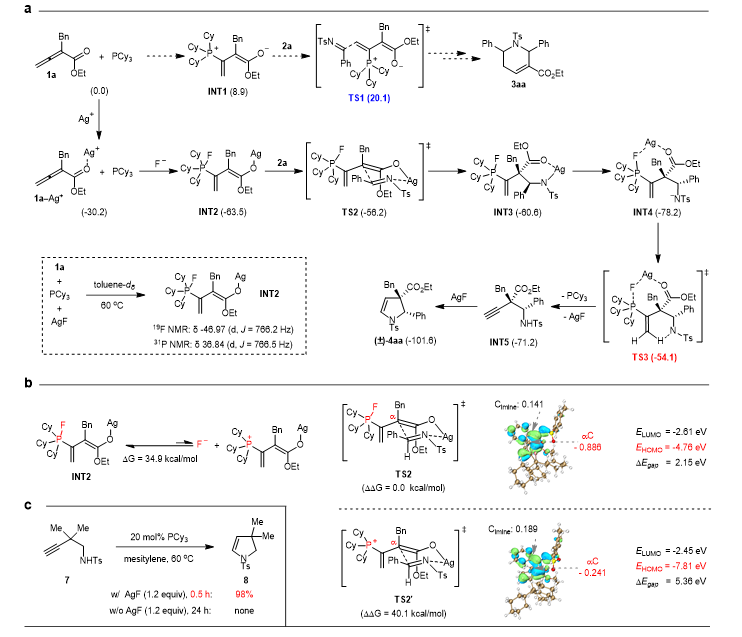
Figure 2: Study on reaction mechanism
The study on the reaction mechanism showed that the power of the AgF additive mainly comes from the strong affinity between Ag+ and the oxygen anion, as well as between F- and the phosphonium cation, which efficiently converts the dipolar ion intermediate A3 into the fluoro-phosphane-enol silver intermediate INT2 (see Figure 2a). Ag+ can further exert its Lewis acidic nature to promote the Mannich reaction of the imine from the original γ position to the α position. Additionally, the fluoro-phosphane structure also favors enhancing the nucleophilicity of the intermediate INT2 (see Figure 2b). AgF also acts as a catalyst for the hydroamination of alkyne, allowing the final cyclization process to be completed smoothly (see Figure 2c).
This research achievement represents a significant breakthrough in the long-standing field of tertiary phosphine catalysis, providing a new development strategy for the field.
The experimental part of this study was mainly completed by Dr. Qian Jinlong, and the theoretical calculations were completed by Dr. Zhou Xiaoyu. Dr. Zhou Xiaoyu and Professor Tong Xiaofeng are the corresponding authors of the paper, with TU as the sole corresponding unit. This research was supported by the National Natural Science Foundation of China.
Full text link: https://www.nature.com/articles/s41467-024-51295-9