Yan Zixin, a 2023-enrolled master’s student in the material engineering program at the School of Materials Science and Engineering of TU, successfully achieved short-range hydrogen spillover by anchoring PtPd alloy nanoclusters on a CeO₂ surface under the guidance of her supervisor. This breakthrough has significantly reduced the reaction energy barrier for acidic hydrogen evolution reactions (HER), offering a novel approach for designing high-efficiency acidic HER catalysts. The findings have recently been published in the authoritative materials journal Angewandte Chemie International Edition (IF=16.1), with Yan Zixin as the first author.
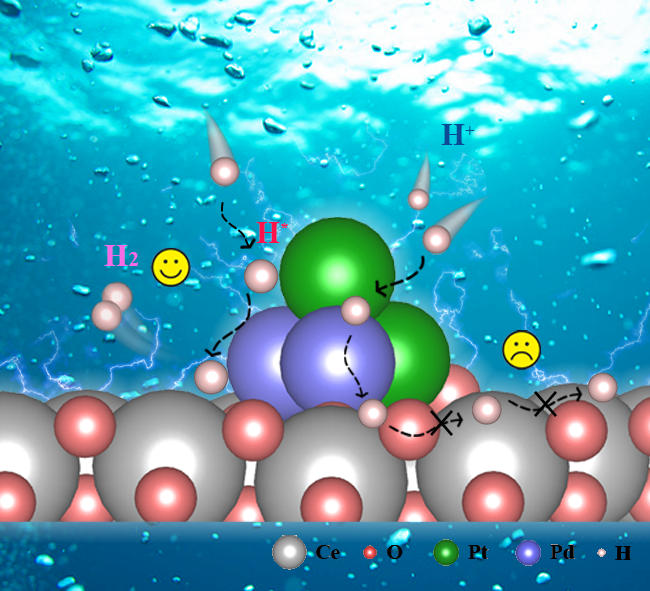
Schematic of the PtPd/CeO₂ Short-Range Hydrogen Spillover Pathway
The hydrogen evolution reaction (HER) is a critical step in water electrolysis for hydrogen production, and developing efficient, stable HER catalysts is a core challenge for large-scale hydrogen energy applications. The hydrogen spillover phenomenon—where hydrogen atoms migrate from highly active metal sites (e.g., platinum, palladium) to adjacent support surfaces or low-activity sites—is recognized as an important mechanism for enhancing HER efficiency. However, its application in acidic environments remains challenging. CeO₂, with its reversible Ce⁴⁺/Ce³⁺ redox pairs and abundant oxygen vacancies, facilitates electron transfer and acts as an “electron pump” to regulate charge distribution, making it an ideal support for electrocatalytic processes. PtPd alloys can not only optimize electronic structures and hydrogen adsorption energies but also reduce platinum usage and costs without sacrificing catalytic efficiency, thus attracting significant interest for inducing hydrogen spillover. However, PtPd alloys typically exhibit strong hydrogen adsorption, leading to high HER overpotentials.
This study explored the HER performance of PtPd alloy nanoclusters anchored on a CeO₂ surface in acidic environments. The research revealed that hydrogen spillover occurred during the HER process. The PtPd alloy optimized the catalyst's charge distribution and amplified the hydrogen spillover effect. Notably, the spillover occurred not from PtPd to CeO₂, but rather from PtPd to the interface. This short-range spillover reduced the energy barrier for hydrogen migration and improved reaction activity. Under acidic conditions, PtPd/CeO₂ exhibited an extremely low overpotential (5.7 mV at 10 mA cm⁻², 32.5 mV at 100 mA cm⁻²) and outstanding catalytic stability (stable operation for at least 400 hours at 100 mA cm⁻²). This study provided crucial insights for designing high-efficiency acidic HER catalysts. Through the short-range hydrogen spillover mechanism, the PtPd/CeO₂ catalyst demonstrated exceptional performance in acidic environments, laying a foundation for large-scale hydrogen energy applications.
Since welcoming its first batch of master’s students in September 2023, the School of Materials Science and Engineering has prioritized the integration of scientific research and education, focusing on synchronous improvement of students’ academic literacy and professional competencies. Graduate students have conducted academic training around socioeconomic needs in new energy and materials fields, yielding a series of research achievements. This study represents an outstanding example of such efforts.
Link to the Paper:
https://onlinelibrary.wiley.com/doi/10.1002/anie.202501964