Professor Ma Da’s team from the School of Pharmaceutical and Chemical Engineering/Advanced Research Institute published recently a research paper titled “Thermally Activated Delayed Fluorescence Enhanced by Macrocyclization” in Science China Chemistry. Dr. Li Shuo is the first author of this paper.
Thermally activated delayed fluorescence (TADF) materials have become a research hotspot due to their broad application prospects in optoelectronic devices, photocatalysis, and phototherapeutics. A key factor in designing TADF materials is ensuring efficient reverse intersystem crossing (RISC) from the lowest excited triplet state (T1) to the lowest excited singlet state (S1). This requires the energy gap (ΔE<sub>ST</sub>) between S1 and T1 to be less than 0.3 eV. To achieve a small energy gap, an effective approach is to construct twisted intramolecular/intermolecular donor-acceptor (D-A) systems.
However, such designs depend on the careful selection of donors and acceptors and their subtle combination, primarily because initial TADF building blocks often have large energy barriers (typically >0.5 eV).
Furthermore, to achieve efficient TADF emission, introducing multiple donors or acceptors is often necessary, but extending the conjugate structure can lead to emission red-shifts.
Therefore, developing new strategies to modulate the energy gap of D-A systems for efficient TADF emission is of great significance.
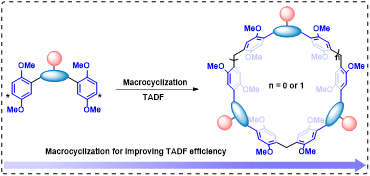
Figure 1 Schematic diagram of constructing efficient TADF via macrocyclization
Based on this, this work proposes a macrocyclization strategy to enhance TADF by linking luminophores via non-conjugated methylene bridges to form macrocycles (Fig. 1). The authors first attached the reaction module (1, 4-dimethoxybenzene) to the fluorophore 5-(9-carbazolyl) isophthalonitrile, then condensed it with paraformaldehyde catalyzed by trifluoroacetic acid to obtain dimeric and trimeric macrocycles.
Analysis of experimental data and theoretical calculations confirmed the TADF properties of these two macrocycles (Fig. 2). Importantly, both macrocycles exhibited higher quantum yields compared to the monomer. This enhancement in TADF efficiency can be attributed to the macrocyclic skeleton structure, which reduces the reorganization energy during structural changes between the S0 and S1 states, effectively decreasing the extent of excited-state energy conversion into non-radiative energy. Importantly, this macrocyclization-enhanced TADF strategy is also applicable to other luminophores, demonstrating good universality (Fig. 3). The integration of macrocyclic chemistry and luminescent materials provides new guidance for designing efficient TADF materials.
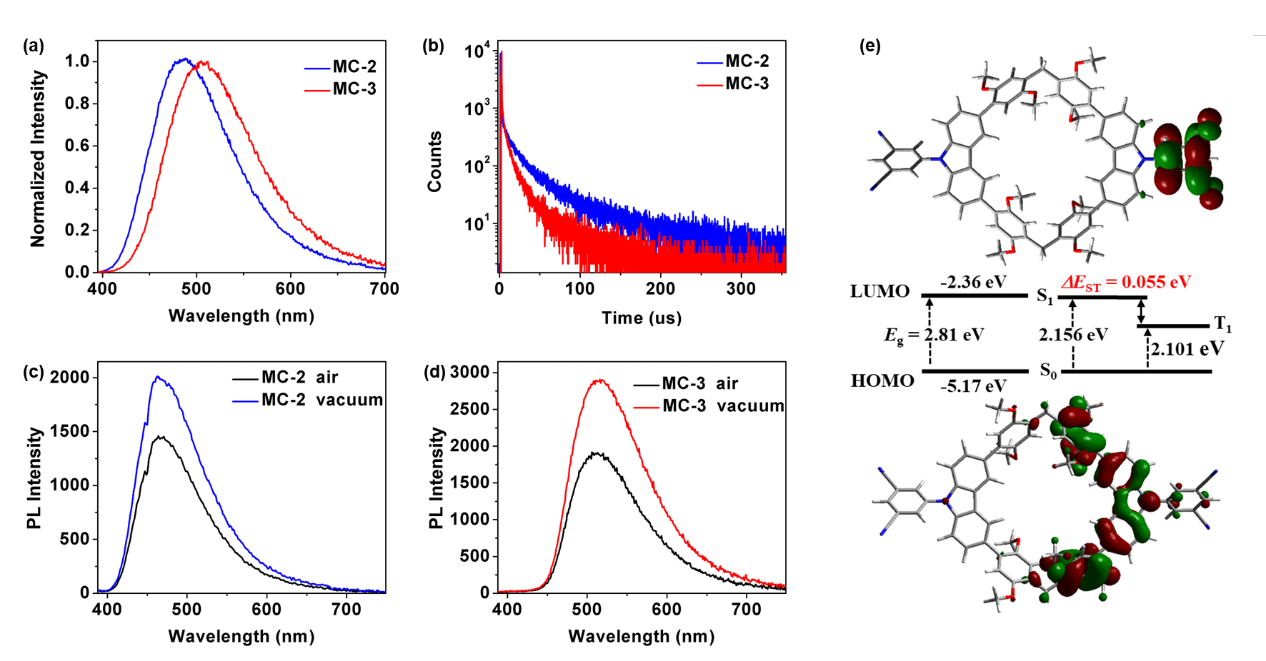
Figure 2 (a) Normalized photoluminescence (PL) spectra of MC-2 and MC-3; (b) Transient PL decay curves of MC-2 and MC-3; (c) PL spectra of MC-2 and (d) MC-3 under air and vacuum conditions; (e) Optimized ground-state geometry, orbital distribution, and energy levels of the dimeric macrocycle MC-2
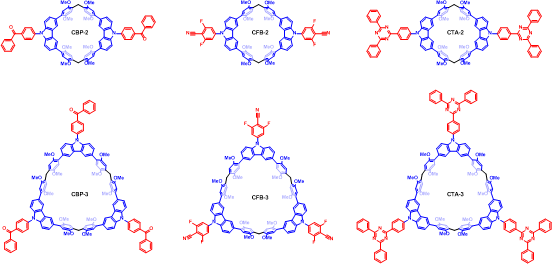
Figure 3 Chemical structures of other macrocycles exhibiting TADF properties
This research was supported by funding from Taizhou University, the National Natural Science Foundation of China, and the Provincial Natural Science Foundation.
Article link: https://doi.org/10.1007/s11426-024-2427-y