A research team led by Wu Jie and Chen Jianqiang from the School of Pharmaceutical and Chemical Engineering/Advanced Research Institute of Taizhou University published recently a paper titled “Enantioselective Alkoxycarbonyl-Lactonization of Alkenes via the Merger of Photoredox and Copper Catalysis” in Advanced Science (Impact Factor: 14.1).
Carboxylic ester structures are fundamental functional groups in organic synthesis, widely found in natural products, pharmaceutically active molecules, and organic synthesis processes. Carboxylic esters can be readily converted into corresponding carboxylic acids or amides. Among the top 200 best-selling small-molecule drugs globally in 2023, 72% contained at least one carboxyl, ester, or amide group.
Therefore, the synthesis of carboxylic ester compounds holds fundamental research value. Current synthetic methods for carboxylic esters are primarily categorized into four types: firstly, esterification of carboxylic acids; secondly, ester synthesis based on Baeyer-Villiger oxidation; thirdly, transition metal-catalyzed carbonylation reactions; and fourthly, synthesis of corresponding carboxylic esters via alkoxycarbonyl radicals. With the recent rise of radical chemistry, reactions involving alkoxycarbonyl radicals have gradually gained increased attention.
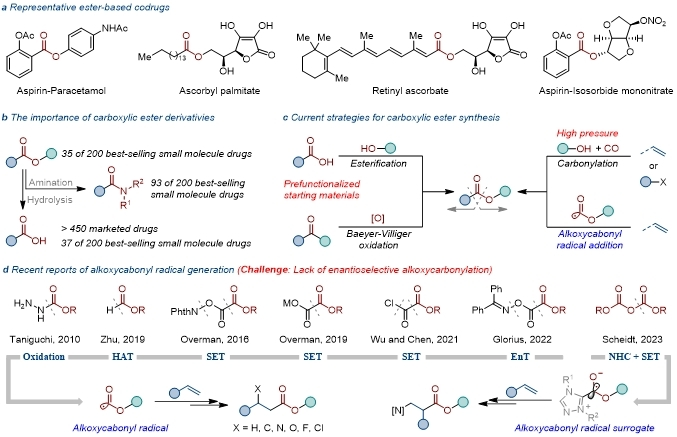
Wu Jie/Chen Jianqiang’s team has long been dedicated to researching alkoxycarbonyl radicals (Nat. Commun. 2021, 12, 5328; Org. Chem. Front., 2021, 8, 6704; Org. Lett., 2022, 24, 642; Green Chem., 2022, 24, 5077; Org. Lett., 2023, 25, 1978; Org. Chem. Front., 2023, 10, 2018; Org. Chem. Front., 2025, 12, 516; Org. Lett., 2025, 27, 427; Org. Chem. Front., 2025, DOI: 10.1039/d5qo00520e; ACS Catal. 2025, 15, 13226).
In 2021, the team first achieved the generation of alkoxycarbonyl radicals via the fragmentation of monoalkyl oxalyl chlorides, using this method to synthesize a series of β-chloro esters (Nat. Commun. 2021, 12, 5328).
Subsequently, in 2022, the group reported the oxidative decarboxylation of monoalkyl oxalates to produce corresponding alkoxycarbonyl radicals, which were then used to synthesize β-fluoro esters (Green Chem. 2022, 24, 5077).
Later, in 2025, the team reported the reductive fragmentation of phthalimide-derived oxalates to generate corresponding alkoxycarbonyl radicals, enabling the synthesis of a series of β-amino acid compounds (Org. Lett. 2025, 27, 427). In the same year, the team also achieved the photo/copper co-catalyzed enantioselective cyano-alkoxycarbonylation of alkenes, synthesizing a series of β-cyano esters (ACS Catal. 2025, 15, 13226).
Building on this prior research foundation, Professor Wu Jie’s team employed a synergistic photoredox/copper catalysis system to achieve the enantioselective alkoxycarbonyl-lactonization of alkenes, synthesizing ester-substituted γ-lactone compounds (Adv. Sci. 2025, e10918).

The aforementioned research received financial support from TU, the National Natural Science Foundation of China, and the Zhejiang Provincial Natural Science Foundation. The theoretical calculations were completed by Dr. Zhou Xiaoyu.
Article link:
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202510918