Dr. Lü Zhongyuan from the School of Pharmacy and the Advanced Research Institute of Biopharmaceuticals, in collaboration with Professor Li Yongquan from the School of Basic Medical Sciences, Zhejiang University, has recently published two research papers in the top-tier international journals Metabolic Engineering and Synthetic and Systems Biotechnology, both ranked Q1 by the Chinese Academy of Sciences.
The papers, titled respectively “The Faucet Knob Effect of DptE Crotonylation on the Initial Flow of Daptomycin Biosynthesis” and “Improving Fidaxomicin Production through ARTP Mutagenesis and Fermentation Optimization in Actinoplanes deccanensis”, list Taizhou University’s School of Pharmacy as a collaborating institution, with Dr. Lü serving as co-corresponding author on both publications.
The first paper points out that daptomycin, a cyclic lipopeptide antibiotic widely used in clinical settings, serves as one of the last-resort treatments for infections caused by multidrug-resistant bacteria, such as vancomycin-resistant enterococci (VRE). Streptomyces roseosporus is the industrial producer of daptomycin, in which the acyl-AMP ligase DptE catalyzes decanoyl acylation to initiate its biosynthesis (Figure 1). The bioactivity of daptomycin is closely related to its decanoyl moiety.
This study proposed that acylation modification of actinomycete proteins functions as a restrictive system that limits the excessive synthesis of secondary metabolites—a mechanism that had not been clearly elucidated before. Using crotonylation as an example, the researchers investigated its role in the daptomycin biosynthetic pathway of S. roseosporus. The results revealed that the key enzyme DptE is crotonylated at K454, and that decrotonylation enhances DptE catalytic activity. Mechanistically, decrotonylation acts like loosening a faucet knob, increasing substrate-channel throughput and the initial flow of daptomycin biosynthesis.
Mutational analysis further showed that substitutions at residues K454, K184, and Q420 significantly enhanced DptE activity, leading to a 132% increase in daptomycin yield. Structural modeling and molecular dynamics simulations demonstrated improved enzyme flexibility and substrate affinity in the mutant variant.
Overall, this work elucidates the “faucet knob effect” of DptE crotonylation on the initiation of daptomycin biosynthesis and establishes a novel strategy to enhance industrial strain productivity through targeted protein decrotonylation. The findings offer valuable insights for researchers in metabolic engineering and microbial natural product biosynthesis, highlighting the potential of enzyme-level post-translational modifications to modulate secondary metabolite production.
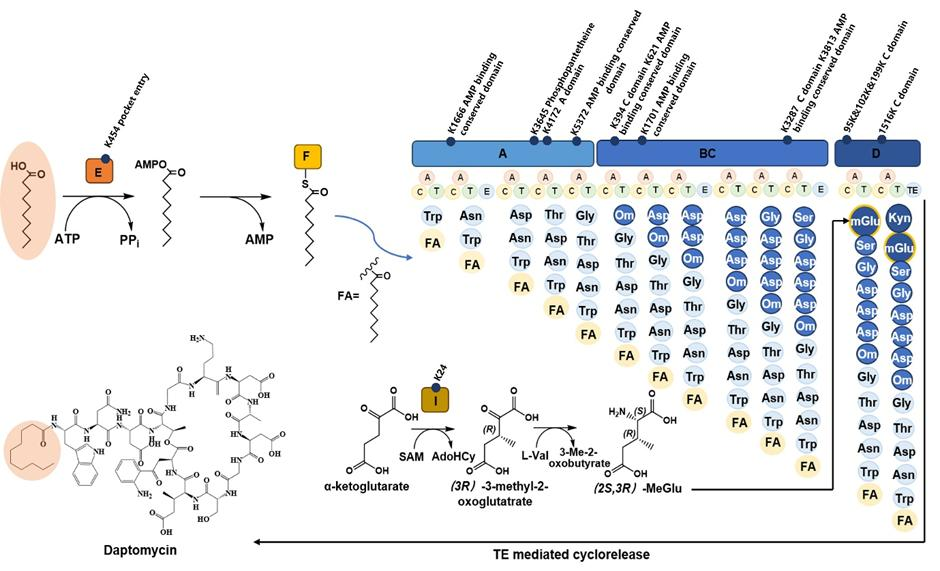
Figure 1 The Daptomycin Biosynthetic Pathway
The second paper points out that fidaxomicin, a macrolide antibiotic, is widely used to treat Clostridioides difficile infection (CDI). It demonstrates significantly higher clinical efficacy than vancomycin and metronidazole. However, its large-scale industrial production remains a significant challenge because of the low fermentation yields.
In this study, the research team chose the strain OE-R1/WT as the starting strain, in which a pathway-specific positive regulatory factor fadR1 was overexpressed. By using the kanR/gusA dual-reporter system and ARTP mutagenesis, they screened a high-yield strain, PA-13, which produced 757.34 mg/L of fidaxomicin, representing a 5.5-fold increase over OE-R1/WT and having enhanced genetic stability. Furthermore, by overexpressing two methyltransferases within the biosynthetic cluster and supplementing with exogenous DMSO, they further increased the production of fidaxomicin to 929.17 mg/L, while reducing the accumulation of the major by-product to 20.9 %. Finally, through the optimization of fermentation strategies at both the shake flask and 15 L fermenter levels, the team achieved a final yield of 3949.05 mg/L in the 15 L fermenter, which represents the highest yield up to date. This study represents the first successful enhancement of fidaxomicin production in Actinoplanes deccanensis to over 3.9 g/L in a 15 L fermenter, establishing a robust foundation for industrial-scale fermentation. Additionally, it provides significant insights for the development of high-yield strains in other actinomycetes.
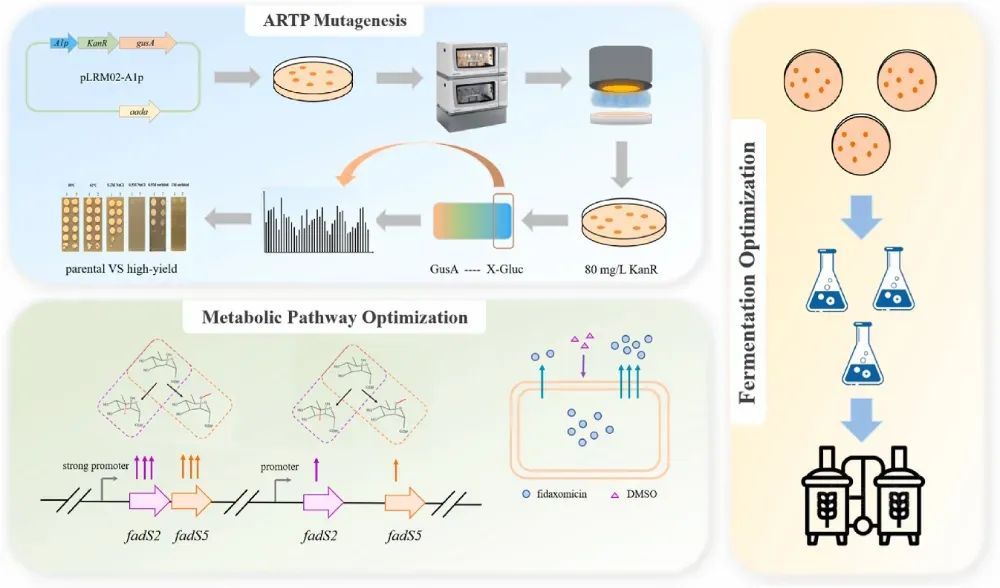
Figure 2 Schematic of Fidaxomicin-Producing Strain Engineering
Link to the first paper:https://doi.org/10.1016/j.ymben.2024.11.003
Link to the second paper:https://doi.org/10.1016/j.synbio.2025.06.002